Her father, a driver in a village near Igatpuri, said there was no reason to refuse the new therapy as his condition was “bad” at the time. “CAR-T saved her,” he told TOI. When the family visited ACTREC, the research block of the Tata Memorial Center in Kharghar earlier in the week, the doctors told them that no cancer cells had been detected in the girl’s blood. “She eats normally for the first time in two years,” she added.
CAR-T cells are a new form of immunotherapy, itself a nascent branch of cancer treatment (see box). It involves reengineering the body’s immune T cells with genetic material so that they selectively target cancer cells for destruction.
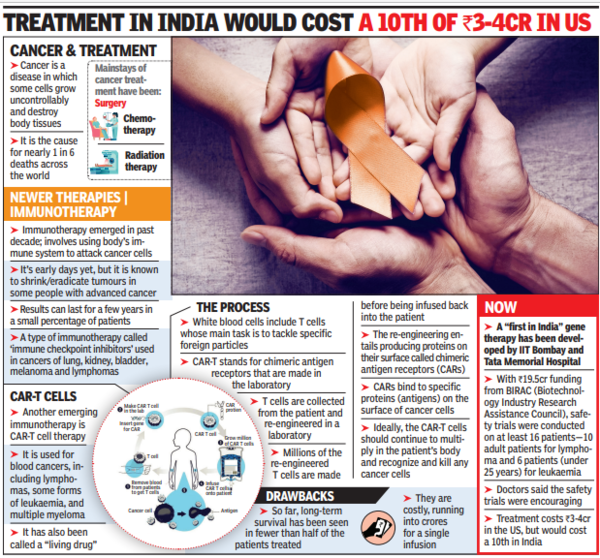
The eight-year-old received treatment as part of safety trials for India’s first in-house manufactured CAR-T cells, a joint effort between IIT-Bombay and Tata Memorial Center, Mumbai (the first patient was infused June 6 2021). The price of this Indian-made therapy will be one-tenth of its cost in the United States, said Dr. Rahul Purwar, an IIT-B scientist who is leading the project.
Last month, the group announced “encouraging” results from the Phase 1 study of 10 patients with lymphoma. Last week, TMC’s Dr. (Surg Cdr) Gaurav Narula announced similar results for the safety study for six leukemia patients at a medical conference in Kochi. “For a group of patients who had no known options, these were very encouraging results and in line with published data from other CAR-T products in other parts of the world,” he said. Doctor Narula. Each of these patients had received three to five lines of therapy, including the previous stem cell transplant, but to no avail.
TMC’s deputy director, Dr Navin Khattry, said that even though it is “still in its infancy” with CAR-T cells, the Indian product has been shown to be safe. “We found that it has low toxicity compared to Western CAR-T cells. For example, 33% of Western patients develop some level of neurological toxicity, but this has not been found in our patients. Furthermore, none of our patients have developed cytokine storms (when the body’s immune system responds too aggressively to infections), ”said Dr. Khattry.
Another eight-year-old who underwent CAR-T infusion more than six months ago is also cancer-free at the moment. Only one of the six patients had no response to CAR-T cells. Another patient, who initially responded well, died 16 months after undergoing a bone marrow transplant.
Doctors hope the 8-year-old and other patients in the future will have the same response as young American Emily Whitehead, who was the first patient in the world to undergo CAR-T cell therapy 10 years ago. She has not needed any therapy thereafter and has been cancer free since 2012.
According to the girl’s father, the doctors first collected her daughter’s blood through a special process for collecting white blood cells. Subsequently, her T lymphocytes were harvested from white blood cells and “transduced” with a viral vector (tools used to deliver genetic material into cells). “This is done to make the T cells express certain antibodies,” said Dr. Narula.
These modified T cells were then multiplied in the IIT Bombay high-tech laboratory. A battery of tests was performed to check for toxicity before it was given to the patient as a single infusion.
“We stayed in ACTREC for 30 days and returned to Igatpuri,” said the father. He has to come back for a check-up in early November.
As for the IIB-TMC team, it is preparing for the second phase of the clinical trial in which 50 patients will be administered CAR-T cells. Purwar, who has since established the ImmunoACT Laboratory, said, “We are also working on gene therapy for various other cancers, including solid tumors,” he said, adding that the goal is to make gene therapy accessible to many more. Indians.
